Introduction: T-Cell Prolymphocytic Leukemia (T-PLL) is a rare, aggressive T-cell malignancy with very poor prognosis. CLL criteria have historically been used for diagnostic and response criteria for T-PLL, but due to the T-PLL's distinct biology and clinical features in 2019 the International Study Group (TPLL-ISG) developed the first consensus criteria for diagnosis and response assessment to harmonize clinical research in T-PLL (Staber et al, Blood, 2019). Diagnostic criteria included both major and minor criteria, while standardized criteria were developed for complete response (CR), complete response with incomplete marrow recovery (CRi), partial response (PR), and stable disease (SD). While the TPLL-ISG consensus criteria are an important step towards uniformity in diagnosis and response assessment, the applicability of these criteria has not been assessed in a real-world population. We evaluated the diagnosis and clinical responses of the OSU T-PLL population using the TPLL-ISG criteria to evaluate the concordance of historical outcomes with previous diagnostic/response criteria.
Methods: We retrospectively evaluated all patients diagnosed with T-PLL at The Ohio State University between 2009-2022. Diagnosis and response were evaluated utilizing the TPLL-ISG criteria. Overall survival (OS), progression-free survival (PFS), and duration of response (DOR) were assessed using Kaplan-Meier methods and log-rank test.
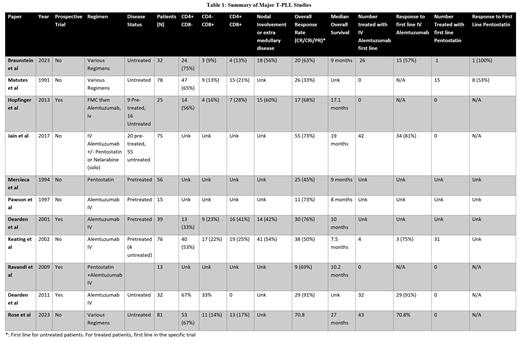
Results: A total of 32 patients, with an average follow-up time of 23 months were evaluated. All patients met the TPLL-ISG criteria for the diagnosis of T-PLL with 29 (91%) meeting major criteria and 22 (69%) meeting both major and minor criteria; concordant with prior studies. 23 patients (72%) were TCL1A positive (by cytogenetics or FISH) and 18 patients (56%) had nodal involvement via PET or CT scan. For all patients, median OS and PFS were 20 months (95% CI 6-45) and 4 months (95% CI 2-17), respectively. Using TPLL-ISG criteria, the overall response rate (ORR) (CR/CRi/PR) to first line treatment was 63% with a median OS of 45 months (95% CI 8-88) and PFS of 26 months (95% CI 4-46). Median DOR was 16 months (range 1-36). The median OS and PFS for patients who achieved a CR were 45 months and 37 months, respectively. For those with a Cri, the OS/PFS was not reached and for those with PR, OS was 7 months and PFS 3 months. The median OS and PFS for patients treated with frontline alemtuzumab (n=26) was 20 months and 4 months and 8 months and 4 months for patients treated with a combination of alemtuzumab/pentostatin (n=4). The ORR for patients treated front-line with alemtuzumab or alemtuzumab/pentostatin was 63% (19/30), with a median OS of 41 months and PFS of 26 months. One patient responded to frontline pentostatin with a DOR of 16 months. The median DOR for any second line treatment (n=7) was 4 months. One patient had a DOR of 4 with alemtuzumab retreatment. Second line pentostatin (n=4) had an ORR of X, and median DOR of 3 months. Patients with CD4+CD8- disease (n=24) had a median OS of 20.4 months and a median PFS of 6 months, while CD4-CD8+ patients (n=3) had an OS of 8 months and PFS of 3 months, and CD4+CD8+ patients (n=4) did not reach median OS or PFS. Nine patients (28%) proceeded to allogeneic stem cell transplantation (allo-SCT). Patients treated with alemtuzumab first line who received allo-SCT had a significantly longer median OS (88 months vs 40 months; p=0.01) and PFS (46 months vs 9 months; p=0.04) compared to those that did not receive an allo-SCT. Response rates in this cohort were similar to published results (Table 1) suggesting no substantial difference between previously utilized response criteria and TPLL-ISG criteria (Table).
Conclusions: No substantial differences in T-PLL response rates or survival were observed when using the TPLL-ISG criteria when compared to historical reports. Patients who had a CR or CRi to alemtuzumab that proceeded with allo-SCT had a significantly prolonged OS and PFS. Patients with CD4-CD8+ T-PLL had inferior survival , but this will need to be validated in a larger sample set. Consistent with previous studies, alemtuzumab produced the best responses, particularly for frontline treatment. Pentostatin provided transient responses as second line treatment but patients inevitably relapsed. Our results confirm the utility of the 2019 consensus criteria and set the basis for larger real-world studies to further assess the outcomes of patients with T-PLL.
Disclosures
Porcu:Kymera: Membership on an entity's Board of Directors or advisory committees; Kyowa: Consultancy; BioGene: Membership on an entity's Board of Directors or advisory committees; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding. Brammer:Verastem: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kymera: Consultancy; Incyte: Research Funding; Dren Bio: Consultancy; Bristol Myers Squibb: Research Funding.